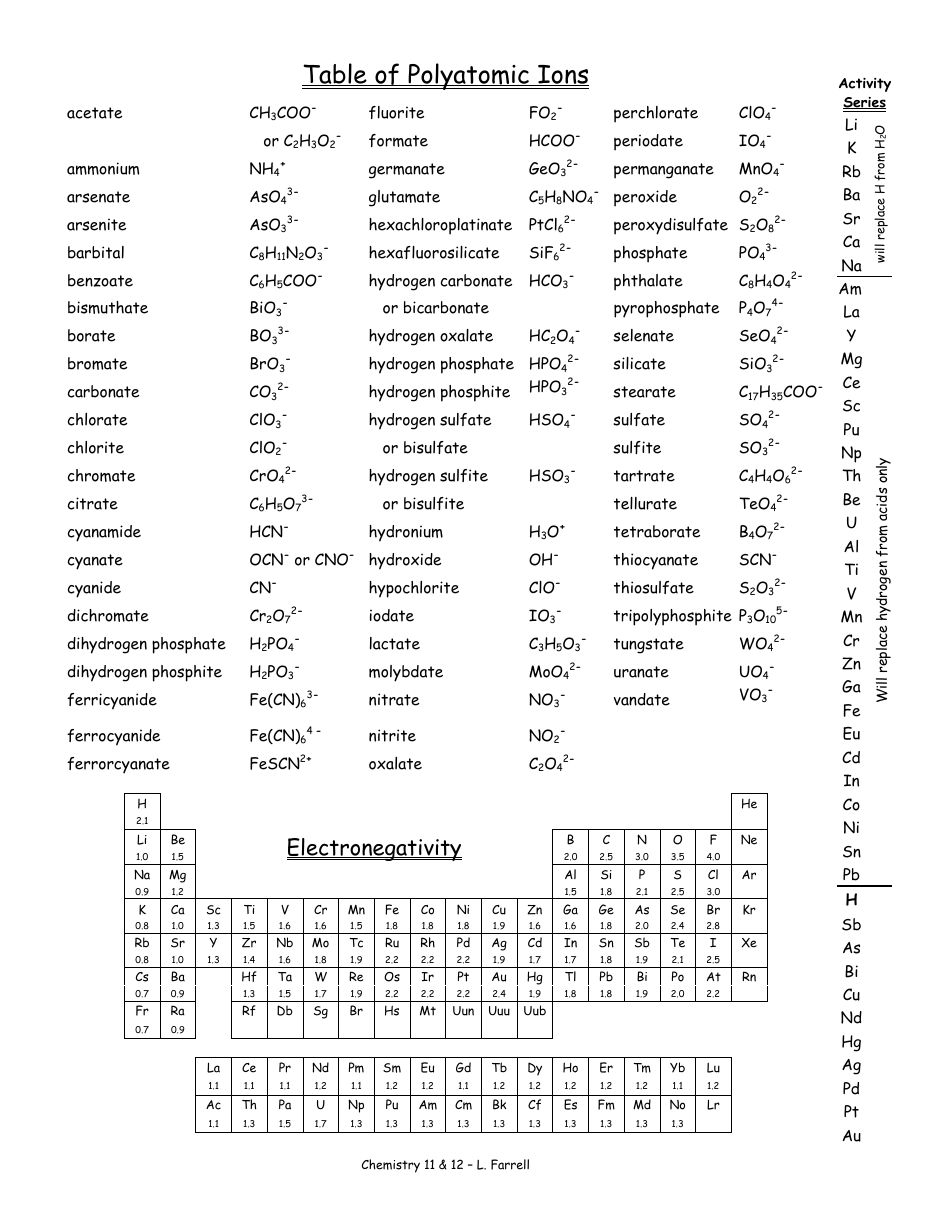
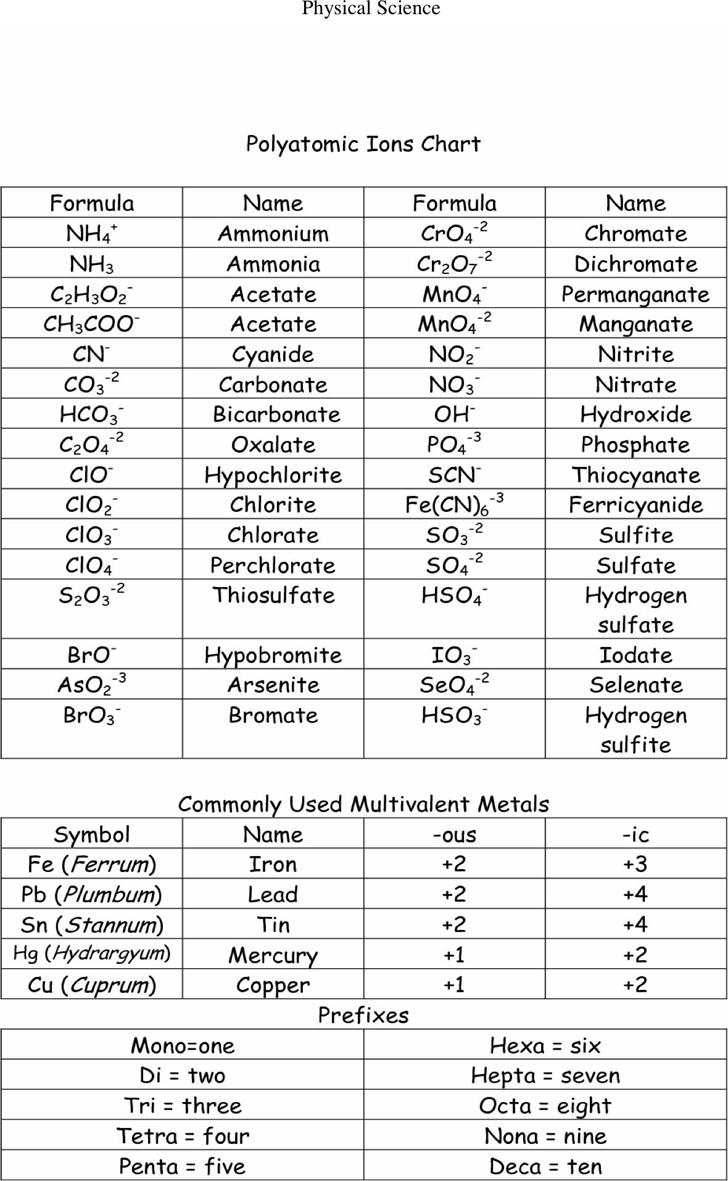
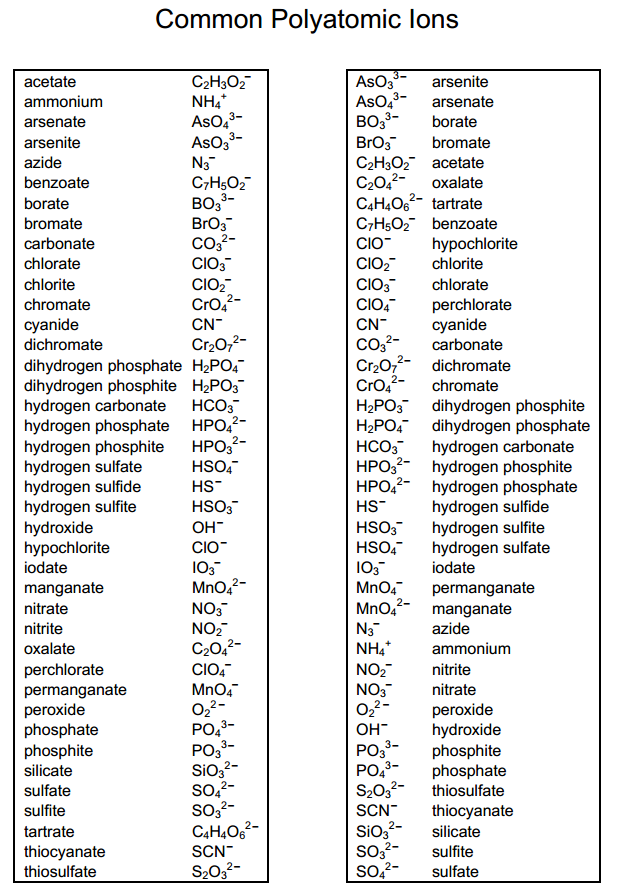
Web other ions consist of a group of atoms with a net charge. Web being familiar with the names, charges, and formulas of the most common polyatomic ions will be helpful for recognizing ionic compounds and predicting their reactivity. Here's a guide to some of the most common examples! ♦ know/ memorize/ recognize names, formulas and charges! Ions with positive charge are called cations.
It has one nitrogen atom and three oxygen atoms and an overall 1− charge. It is used to help identify and write the formulas for these ions in chemical compounds. Let's explore some of the most common polyatomic ions and learn how to write chemical formulas for compounds containing these ions. A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. For example, \(\ce{no3^{−}}\) is the nitrate ion;
The polyatomic ions remain intact, and parentheses may be required when using subscripts. Each entry contains the ion's name, molecular formula and chemical structure. It has one nitrogen atom and three oxygen atoms and an overall 1− charge. Web this document lists and describes many common polyatomic ions, including their chemical formulas and charge. Web this is a list of some of the most common polyatomic ions.
Memorize all of the binary compounds: The polyatomic ions remain intact, and parentheses may be required when using subscripts. It is worth committing the polyatomic ions to memory, including their molecular formulas and ionic charge. Web this document lists and describes many common polyatomic ions, including their chemical formulas and charge. Web this is a list of some of the most common polyatomic ions. It has five atoms (one nitrogen and four hydrogens) that share a charge of +1. Ions made up of more than one atom are known as polyatomic ions. It also provides some rules for naming polyatomic ions based on adding or removing oxygen and hydrogen atoms. Web on the other hand, if an ion is made up of two or more atoms, it can be referred to as a polyatomic ion or a molecular ion. Since these ions are composed of multiple atoms covalently bonded together, they are called polyatomic ions. For example, ammonium chloride is nh 4 cl and ammonium sulfide is (nh 4) 2 s. Ions with positive charge are called cations. The following table lists some of the common polyatomic ions. Memorize all of the polyatomic ions: Web other ions consist of a group of atoms with a net charge.
There Are 4 Exercises To Practice, Plus Complete Instructions, In The 5 Page Packet.
It is worth committing the polyatomic ions to memory, including their molecular formulas and ionic charge. Here's a guide to some of the most common examples! Memorize all names and formulas on back. It is used to help identify and write the formulas for these ions in chemical compounds.
It Has Five Atoms (One Nitrogen And Four Hydrogens) That Share A Charge Of +1.
Web ap chemistry polyatomic list. Web polyatomic ions are charged groups of atoms. Ions with positive charge are called cations. Web list of polyatomic ions * you do not need to memorize these metasilicate sio32— nitrate no3— nitrite no2— oxalate c2o42— perbromate bro4— hypobromite bro— perchlorate clo4— hypochlorite clo— periodate io4— hypoiodtite io— permanganate mno4— peroxide o22— phosphate po43— phosphite po33— *phosphonium ph4+.
Web This Is A List Of Some Of The Most Common Polyatomic Ions.
Web the common polyatomic ions chart provides a list of common ions with their respective formulas. Each entry contains the ion's name, molecular formula and chemical structure. It has one nitrogen atom and three oxygen atoms and an overall 1− charge. Web other ions consist of a group of atoms with a net charge.
Web This Is A List Of Some Of The Most Common Polyatomic Ions.
For example, ammonium chloride is nh 4 cl and ammonium sulfide is (nh 4) 2 s. Web this document lists and describes many common polyatomic ions, including their chemical formulas and charge. Ions made up of more than one atom are known as polyatomic ions. It is worth committing the polyatomic ions to memory, including their molecular formulas and ionic charge.