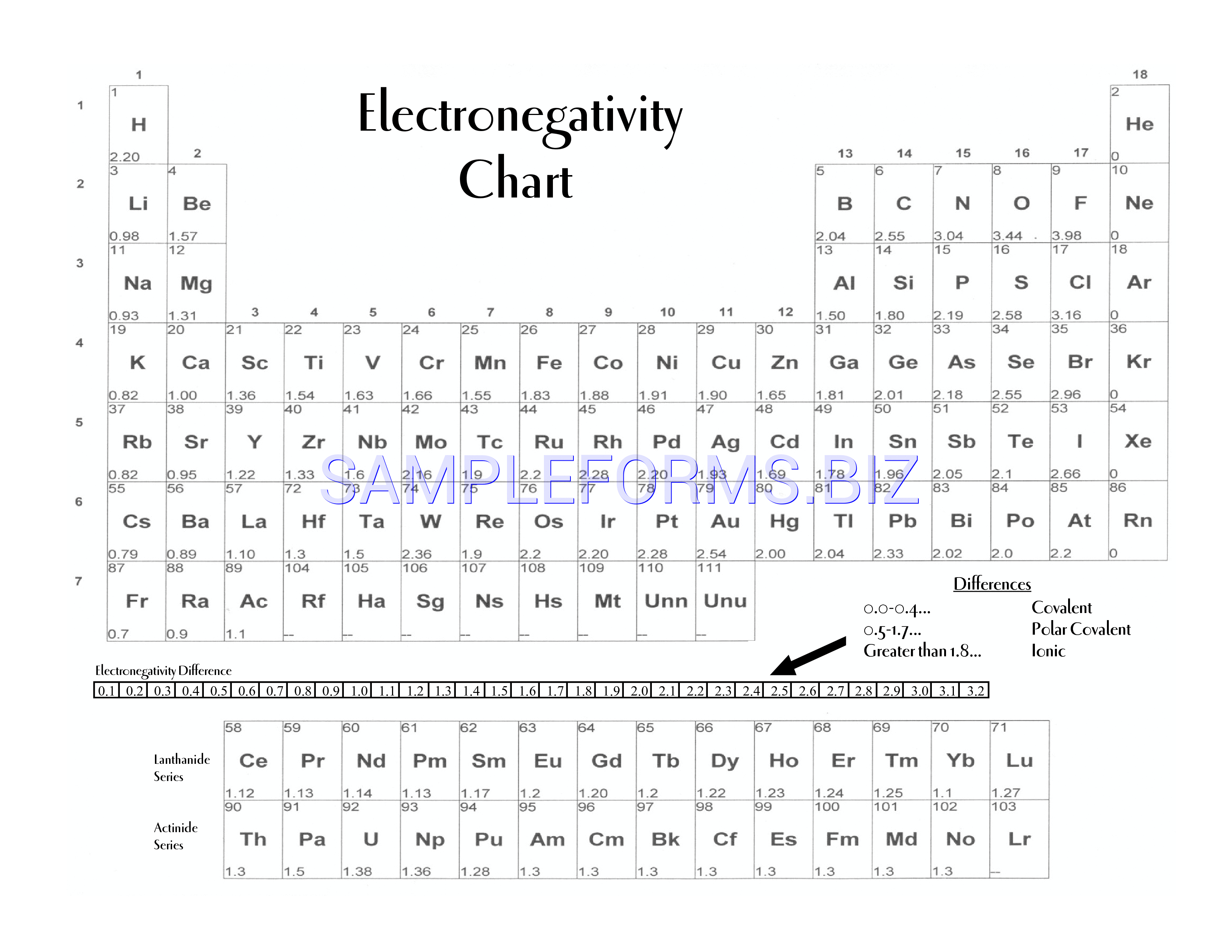
Electronegativity, image χ, is a substance property that portrays the inclination of an iota to pull in a mutual match of electrons (or electron thickness) towards itself. This color printable periodic table contains the element number, element symbol and electronegativity. This table displays the linus pauling derivation of electronegativities. But if you want to see the electronegativities of all the 118 elements, then visit: 1 h hydrogen 2.20 3 li lithium 0.98 4 be beryllium 1.57 11 na sodium 0.93 12 mg magnesium 1.31 19 k potassium 0.82 20 ca calcium 1.00 21 sc scandium 1.36 22 ti titanium 1.54 23 v
These increasing electronegativity chart templates have values for electronegativity which run from o to 4. An atom with high electronegativity pulls electrons strongly, while an atom with low electronegativity drags them weakly. We use a quantity called electronegativity to estimate whether a given bond is nonpolar covalent, polar covalent, or ionic. In chemistry, electronegativity is a test to get to know how strongly an atom attracts electrons in a bond. This table is a list of electronegativity values of the elements.
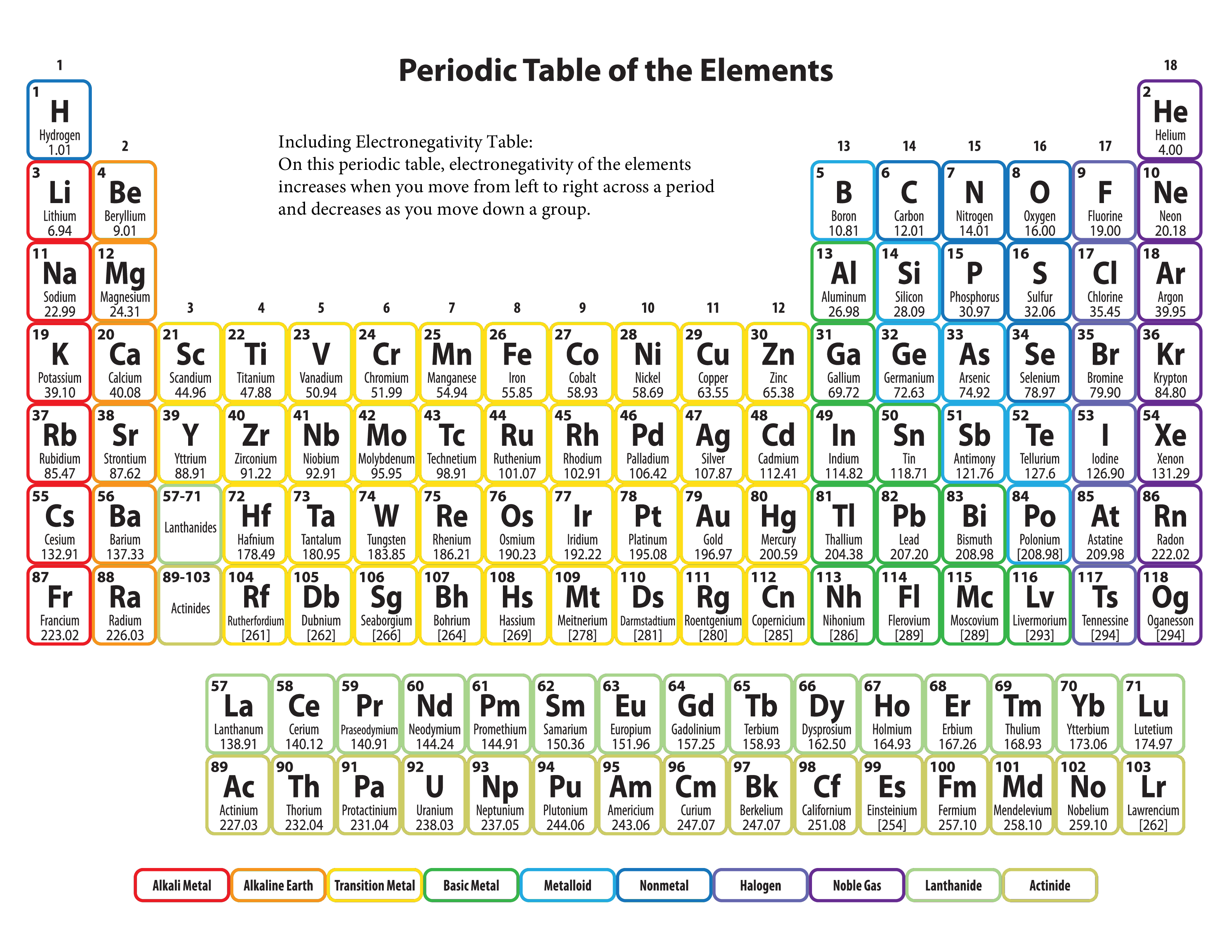
These are the electronegativities of first 20 elements of the periodic table. This is a collection of periodic tables that you can print and use. This electronegativity chart pdf is a useful reference tool for the elements and their electronegativity values. Find a periodic table of all elements with respective eletronegativities. It can also be used to predict if the resulting molecule will be polar or nonpolar.
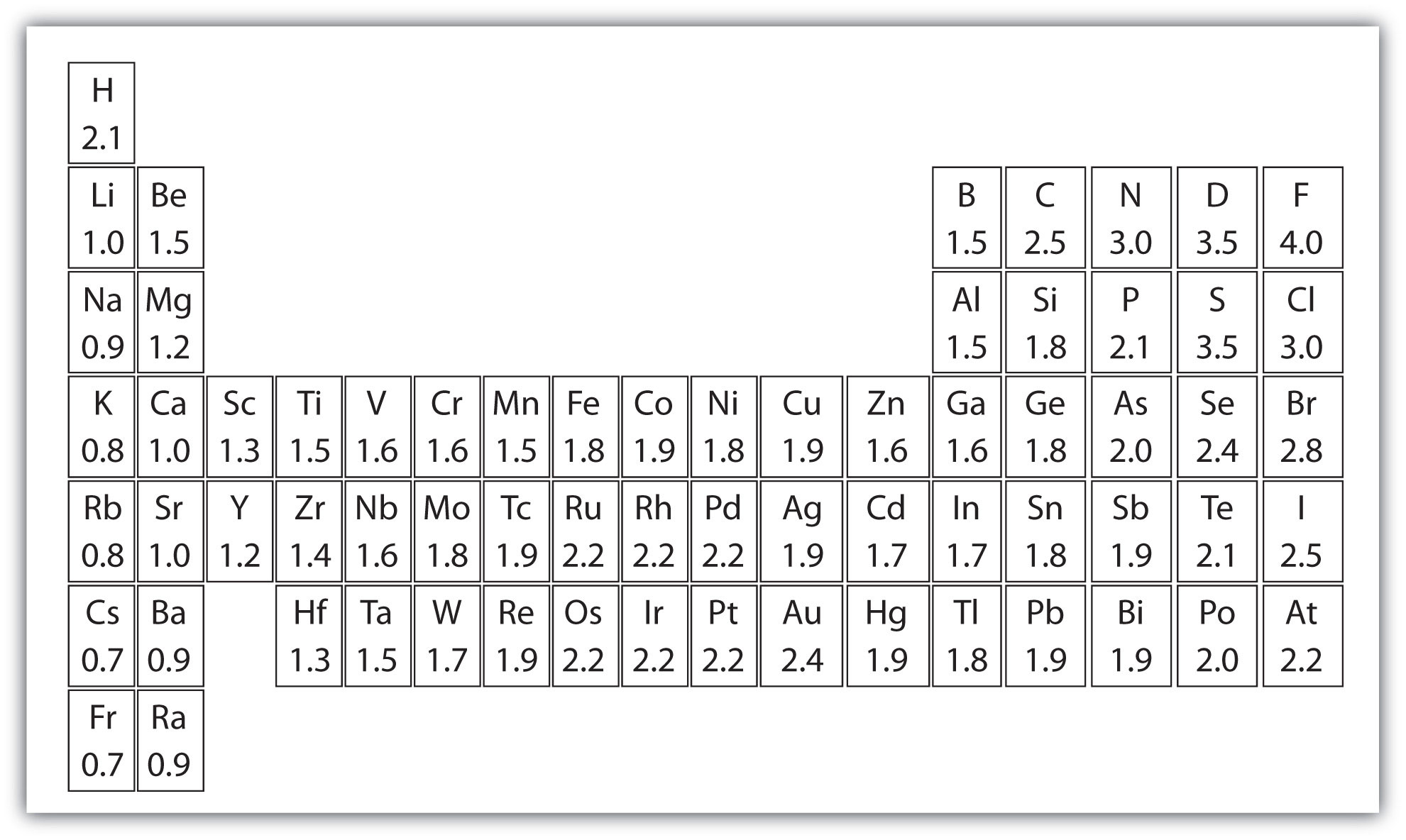
This table is a list of electronegativity values of the elements. Print out a pdf version of electronegativity chart to study and for reference. Every other element's electronegativity has been scaled accordingly. Fluorine, the most electronegative element, has arbitrarily been given a value of 4.0. Electronegativity 17 atomic number cl symbol chlorine name 3.16 electronegativity (pauling scale) 1 h hydrogen 2.2 1 1 2 he helium 18 3 li lithium 0.98 2 4 be beryllium 1.57 2 5 b boron 2.04 13 6 c carbon 2.55 14 7 n nitrogen 3.04 15 8 o oxygen 3.44 16 9 f fluorine 3.98 17 10 ne neon 11 na sodium 0.93 3 12 mg magnesium 1.31 13 al aluminum 1.61. This page has a printable electronegativity table, also called an electronegativity chart. Perfect for printing large scale prints for the classroom or house art. Periodic table of electronegativities 1 h 2.1 2 he 3 li 1.0 4 be 1.5 5 b 2.0 6 c 2.5 7 n 3.0 8 o 3.5 9 f 4.0 10 ne 11 na 0.9 12 mg 1.2 13 al 1.5 This color printable periodic table contains the element number, element symbol and electronegativity. Earhart 11/22/2016 1 h 2.20 3 li 0.98 4 be 1.57 5 b 2.04 6 c 2.55 7 n 3.04 8 o 3.44 9 A molecule’s electronegativity is influenced by the two its nuclear number and the separation at which its valence electrons live from the charged core. Values for electronegativity run from 0 to 4. The periodic table of the elements (with electronegativities) element name. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. They also include varying types of data, such as boiling or melting points, electronegativity, valence, density, and more.
The Electronegativity Chart Describes How Atoms Can Attract A Pair Of Electrons To Itself And By Looking At The Chart You Can Easily Determine The Electronegativity Values Of All The Elements.
This electronegativity chart pdf is a useful reference tool for the elements and their electronegativity values. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Print out a pdf version of electronegativity chart to study and for reference. 1 h hydrogen 2.20 3 li lithium 0.98 4 be beryllium 1.57 11 na sodium 0.93 12 mg magnesium 1.31 19 k potassium 0.82 20 ca calcium 1.00 21 sc scandium 1.36 22 ti titanium 1.54 23 v
Electronegativity 17 Atomic Number Cl Symbol Chlorine Name 3.16 Electronegativity (Pauling Scale) 1 H Hydrogen 2.2 1 1 2 He Helium 18 3 Li Lithium 0.98 2 4 Be Beryllium 1.57 2 5 B Boron 2.04 13 6 C Carbon 2.55 14 7 N Nitrogen 3.04 15 8 O Oxygen 3.44 16 9 F Fluorine 3.98 17 10 Ne Neon 11 Na Sodium 0.93 3 12 Mg Magnesium 1.31 13 Al Aluminum 1.61.
The electronegativity definition and electronegativity trend are described below. A molecule’s electronegativity is influenced by the two its nuclear number and the separation at which its valence electrons live from the charged core. This table displays the linus pauling derivation of electronegativities. It can also be used to predict if the resulting molecule will be polar or nonpolar.
Electronegativity Is The Tendency Of An Atom To Attract A Pair Of Electrons In A Chemical Bond.
This color printable periodic table contains the element number, element symbol and electronegativity. Earhart 11/22/2016 1 h 2.20 3 li 0.98 4 be 1.57 5 b 2.04 6 c 2.55 7 n 3.04 8 o 3.44 9 This table is a list of electronegativity values of the elements. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons.
But If You Want To See The Electronegativities Of All The 118 Elements, Then Visit:
This page has a printable electronegativity table, also called an electronegativity chart. Perfect for printing large scale prints for the classroom or house art. These increasing electronegativity chart templates have values for electronegativity which run from o to 4. Fluorine, the most electronegative element, has arbitrarily been given a value of 4.0.





:max_bytes(150000):strip_icc()/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png)
